A question that often comes back to the PARTENAIR standard, what becomes of the pressure dew point when the pressure changes?
Reminder: The dew point is, at a given temperature, the saturated water vapor content in the air regardless of the pressure. A volume of 1 m3, under a pressure of 7 or 30 bars at a temperature of 35 ° C and 100% saturated in humidity, will always contain - in both cases - 39.2 grams of water vapor.
If the pressure is lowered, the air volume increases but the water content does not change. So the dew point decreases.
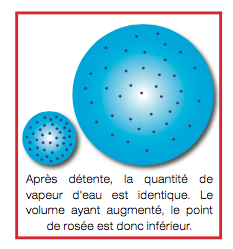
The volume having increased the dew point is therefore lower
If the pressure is increased (in the case of a booster for example) the volume of air decreases. The water content remains the same, so the dew point increases.
Remember: Pressure reduction = dew point reduction.
A dew point of + 3 ° C under 7 bars becomes a dew point of -22 ° C when the air is relaxed to atmospheric pressure.
Conversely, a pressure dew point of + 3 ° C at 7 bars becomes a pressure dew point of + 23 ° C once the air is boosted to 30 bars.


